Associate Professor Neils Jonassen authored a bi-monthly static column that appeared in Compliance Engineering Magazine. The series explored charging, ionization, explosions, and other ESD related topics. The ESD Association, working with In Compliance Magazine is re-publishing this series as the articles offer timeless insight into the field of electrostatics.
Professor Jonassen was a member of the ESD Association from 1983-2006. He received the ESD Association Outstanding Contribution Award in 1989 and authored technical papers, books and technical reports. He is remembered for his contributions to the understanding of Electrostatic control, and in his memory we reprise “Mr. Static”.
~ The ESD Association
Reprinted with permission from: Compliance Engineering Magazine, Mr. Static Column Copyright © UBM Cannon
Over the years, there have been numerous reports of explosions in grain silos, of oil tankers blowing up during tank washing, of patients being killed during an operation by a pressure wave set off by an ignition of the anesthetic gas, of everything from minor accidents in the laboratory or kitchen to disasters in space vehicles. It’s an interesting story in itself how the number of static-caused explosions seems to have dwindled over the last two decades, but we will leave that one to another discussion. Instead, we will look into what a discharge is and what sometimes makes it incendive—that is, capable of causing an explosion.
Decay and Discharge
A charged body may lose its charge in two ways. First, let’s suppose the body is a conductor. If it is connected to ground by a path containing mobile charge carriers, the charge will apparently leak away in a current. This is what happens in any wrist strap or surface layer of a topical antistat. If, by contrast, the charged body is a true insulator, this process can only take place if the body is totally immersed in a conductive fluid—in practice, always ionized air. In this case the body doesn’t really lose its charge. Rather, the field is neutralized by oppositely charged ions attracted from the fluid. This is called charge decay.
The decay current is driven by the field from the charge to be neutralized, but all the field does is move existing charge carriers. The only effect of a decay current (apart from neutralizing the charge and field) is a dissipation of heat, as given by Joule’s law.
The other way by which a body may “lose” its charge, totally or partly, is through an electrostatic discharge. A discharge happens if the field from a charge is high enough to cause ionization in the surrounding medium. The difference between decay and discharge is primarily that, in the discharge process, the charge carriers are created by the field, and the development of the process may be much more dramatic than in decay.
In a casual context, electrical discharges are often called sparks. It is, however, more practical to reserve this name for a special kind of discharge, namely that taking place between well-rounded conductors at different potentials.
Types of Discharge
Bowing to tradition and convenience, we may divide electrical discharges into three sometimes-overlapping groups: corona, spark, and brush discharges.
Corona Discharge. If the field strength in front of a sharp point of a conductor exceeds the breakdown field strength for the medium (air, for instance), a corona discharge will take place. This may happen if a conductor with sharp protrusions is given a high voltage, the critical value of which depends upon the geometric conditions, like distance to grounded surroundings. But it may also happen if a grounded, sharp conductor (at zero voltage) is brought near a charged object, like a piece of plastic that has been rubbed. This event demonstrates that it does not take a high voltage to cause a discharge, only a high field strength (see Figure 1).

Figure 1: Corona discharge
In a corona discharge, the ionization is limited to a small region around the electrode, where the breakdown field strength is exceeded. In the rest of the field, we have just a current of slow-moving ions and even slower-moving charged particles finding their way to some suitable counter electrode, such as the walls of the room.
A corona discharge is also called a silent discharge. It may be maintained as long as the breakdown field strength is exceeded in some region—that is, as long as the voltage of the electrode or the charge density of the charged insulator is high enough.
Spark Discharge. At the other extreme of the discharge range, we have the spark. This kind of discharge may take place between two well-rounded conductors at different potentials, one of them often grounded (see Figure 2). Again, the discharge starts at a point where the breakdown field strength is exceeded. But in contrast to the corona discharge, in a spark the ionization takes place all the way between the two electrodes.
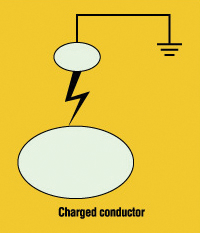
Figure 2: Spark discharge
If the electrodes are connected to a voltage supply, the discharge may turn into a continuous arc, but in the normal case of a spark from an insulated
conductor, the discharge is a very fast process, where energy given by the equation
![]()
is dissipated in the narrow discharge volume.Here C is the intercapacitance of the two electrodes, V their potential difference.
Brush Discharge. In between the corona discharge and the spark is the brush discharge, which may take place, for example, between a charged material and a normally grounded electrode with a radius of curvature of some millimeters. If a brush discharge is maintained over longer periods, it may appear as irregular luminescent paths (see Figure 3).
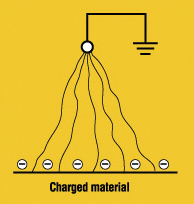
Figure 3: Brush discharge
Almost all discharges from insulators are brush discharges, like the crackle that you hear when you pick up a charged photocopy or that you feel when you pull a sweater over your head. Only if the discharge comes from a heavily charged, thin sheet of an insulator backed by a grounded conductor (stemmed branched brush discharge) can the discharge have something close to the properties of a spark.
Incendivity
For our purposes here, the difference between the various types of discharges, as just described, lies primarily in their different incendivity —that is, the ability of a discharge to cause ignition or combustion. If we have a mixture of, say, oxygen (O2) and diethyl ether ([C2H5]2O), the molecules may react with each other if they get into a close-enough encounter, forming water and carbon dioxide. For this to happen, a certain amount of energy has to be delivered in a sufficiently small volume and in a sufficiently short time. The amount of energy depends strongly upon the gas mixture, both in terms of the types of components as well as their relative concentrations.
Figure 4 shows the ignition energies for diethyl ether vapor mixed with either pure oxygen or atmospheric air. For a concentration of approximately 16% ether vapor in pure oxygen, it takes only about 1 μJ to start an explosion. For ether vapor in atmospheric air, the minimum ignition energy is about 0.2 mJ for a concentration of about 6% ether vapor.

Figure 4: Ignition energy for diethyl ether mixtures
Although the curves in Figure 4 are developed specifically for diethyl ether, they are fairly typical for a wide range of vapors of organic compounds, aliphatic as well as cyclic. Consequently, the value of 0.2 mJ may be regarded as a rule-of-thumb lower-energy limit for vapor-air mixtures. Thus, whether an electrostatic charge may cause an ignition in a given environment depends on whether the discharge may deliver an energy of more than 0.2 mJ (or the relevant specific value) in a small-enough volume and in a sufficiently short time.
How incendive, then, are the various types of discharge we›ve discussed? The rate and density of the energy dissipated in corona discharges will always be too low to initiate an ignition—in other words, they are not incendive under any circumstances. In brush discharges, the total energy may easily be high enough, but in most cases either the rate or the density of the energy dissipation is too low to cause an ignition. It is nonetheless possible to create such charging and discharging conditions that a brush discharge may cause ignition in a mixture of common organic vapors and atmospheric air. But it should be stressed that such conditions are very rarely, if ever, encountered by accident. Therefore, we may conclude that brush discharges, and thus discharges from insulators, have very low incendivity.
It›s a completely different story with sparks. Again, sparks are discharges between rounded conductors (one of them, often, a grounded object) at different potentials. As already suggested, such a system may be characterized electrostatically by the intercapacitance (or partial capacitance) C of the electrodes. If the voltage difference between the electrodes is V, an energy W given by the equation
![]()
will be stored in the system. If a spark occurs, almost all of this energy will be rapidly dissipated in the narrow discharge volume. If the discharge occurs in an explosive atmosphere, ignition may result.
By way of example, let’s examine a fairly ordinary situation. A person with a capacitance of, say, 200 pF walks across an insulating carpet or takes off a sweater (or does both). She hereby gets charged to a voltage of 2000 V and is loaded with an electrostatic energy of 0.4 mJ. She then starts to remove her nail polish using a solvent that is mainly acetone, (C2H5)2CO. This solvent has a minimum ignition energy like that of diethyl ether, around 0.2 mJ in atmospheric air. If she next touches a grounded item and causes a spark in the vicinity of the open bottle of polish remover, will she cause an explosion?
Most likely not. If we look again at Figure 4, we notice that the curve corresponding to atmospheric air is very narrow. This means that as soon as you move just slightly outside the most easily ignited mixture (6% ether), the necessary energy is much higher. It is therefore possible only in a very small region to cause the acetone vapor to ignite by a 0.2-mJ spark.
On the other hand, somewhere between the surface of the acetone, where the mixture is too rich, to perhaps a couple of feet away, where the mixture is too lean, we’ll find the most volatile mixture. If our polish-removing person is very unlucky, that’s where she may draw a spark.
Explosion-Safe Voltage
It is fairly safe to assume that an electric discharge disseminating an energy less than the minimum ignition energy Wmin ~ 0.2 mJ in atmospheric air is not incendive, no matter what explosive vapors are present. For a capacitive system—that is, an insulated conductor—with the capacitance C, we may thus define an “explosion-safe voltage” Vex as
![]()
In the case of our friend with the polish remover, we find the theoretical safe voltage to be 1400 V.
The concept of a safe voltage level refers only to explosion risks. When dealing with electronics, the acceptable voltage levels are often considerably lower. And needless to say, the safe voltage concept can also not be applied to charged insulators. Why not? Simply because there is no such thing as the voltage of an insulator. ![]()
 |
Niels Jonassen, MSc, DSc worked for 40 years at the Technical University of Denmark, where he conducted classes in electromagnetism, static and atmospheric electricity, airborne radioactivity, and indoor climate. After retiring, he divided his time among the laboratory, his home, and Thailand, writing on static electricity topics and pursuing cooking classes. Mr. Jonassen passed away in 2006. |
