A Rigorous Process to Achieve Core and Global Market Access
According to the U.S. Consumer Product Safety Commission (CPSC), at least 41 Americans were killed and about 133,000 injured between 2017 and 2019 in incidents tied to e-scooters, e-bikes, and hoverboards.1 Ten companies were forced to recall approximately 500,000 hoverboards after the CPSC received about 100 reports of the lithium-ion battery packs that power hoverboards overheating, sparking, smoking, catching fire, or exploding.2
While these examples may represent a small segment of concern, their occurrence highlights the importance of product regulatory compliance and the consequences of failing to integrate compliance considerations into the design, development, production, and distribution of a wide range of products.
Background and Definitions
The world is full of regulations. Local, state, national, and international jurisdictions have in place a variety of regulations and regulatory compliance requirements addressing user safety and health, energy use, environmental issues, and other product-related considerations.
The product regulatory compliance process encompasses all of these aspects in the regulation of end products, components, materials, systems, and processes. It chiefly consists of testing an end product to assess its compliance with applicable requirements and receiving certification from a regulatory agency or a self-declaration by the manufacturer that the end product meets these requirements.
Typically, these requirements apply to products that utilize modern electronic technologies. However, many product regulatory requirements address various health, environmental, and safety issues specific to other types of products, including foods and grains, drugs, oils, chemicals, fabrics, cosmetics, etc.
An original equipment manufacturer (OEM) is obligated to test its products to determine their conformity with the applicable standards mandated by the regulatory authority of the country in which the products will be sold or marketed. In many cases, OEMs are also required to obtain independent verification of conformity and receive certification or other form of approval prior to shipping the product to that market. A copy of the certification or other evidence of product approval is generally required to accompany the product when shipped.
Product regulatory compliance is achieved at the product or stock-keeping unit (SKU) level, and marking verifying that compliance is generally required to be visible on the product. In some cases, product regulatory compliance requirements are also applicable to critical components within the product or spare parts that accompany the product when sold. Generally speaking, achieving compliance with component level regulations is the responsibility of the component supplier, and test data verifying component compliance is included in documentation submitted in compliance declarations covering the actual end product.
Relevant regulatory requirements can vary based on a country or jurisdiction, the industry, or the technology used. For electrical and electronic systems, devices, and components, requirements may include, but are not limited to, issues related to safety, electromagnetic compatibility (EMC), radio, telecommunications, energy efficiency, environmental, quality, performance, etc. Further complicating the compliance picture, individual technical requirements can vary from country to country, contributing to the challenges of achieving global regulatory compliance.
Rapid advances in technology are bringing many benefits to humankind, but they are also accompanied by threats and vulnerabilities. As businesses are rapidly digitizing records and processes through the use of SaaS products, and as new devices increasingly rely on the use of radio for the internet connectivity, the field of Cybersecurity is quickly advancing to protect businesses and people from digital attacks. Product Regulatory Compliance globally is making a foray into cybersecurity as a next frontier that must be tackled and is aggressively advancing to include cybersecurity as one of the critical disciplines of conformance.
In addition to compliance certifications and approvals issued by regulatory authorities, several industry special interest groups (SIGs), consortiums, and alliances, such as the Wi-Fi Alliance, the Zigbee Alliance, and the LoRA Alliance, offer product or technology-specific approvals that allow the use of their logo or other identification on products that have been reviewed and verified for compliance with their technology-specific requirements.
Medical devices and instruments used for important functions are also held to rigid performance standards. A few examples of devices and instruments that must meet performance-related standards include pulmonary and respiratory systems, ventilators, blood pressure measurement devices, intravenous instruments, pediatric tracheostomy tubes, feeding systems, culture media used in microbiology laboratories, certain materials used in the practice of dentistry, patient transfer chairs, and sterile containers, etc.
Many government agencies, including those overseeing aviation and military systems and applications, may also require conformity with specialized performance and quality standards that may not fall within the typical definition of product regulatory compliance but which must be addressed nonetheless. For example, “The U.S. Internal Revenue Service released Notice 2015-4 which specifies the performance and quality standards that small wind turbines must meet in order to qualify for the 30% investment tax credit, and which requires that small wind turbine models be certified ……” 3
Consumer and enterprise products requiring access to telecommunications networks operated by mobile phone carriers may have to comply with the requirements developed by Telcordia, a telecommunications standards body. In addition to the Telcordia requirements, carrier-specific requirements are often imposed by network providers like Verizon, AT&T, T-Mobile, Vodafone, Orange, Telstra, etc. Typically, collaborating with the network providers to conduct tests and satisfy such requirements also becomes the responsibility of compliance engineers.
Finally, large e-commerce retailers and distributors may have their own requirements applicable to the products that they procure for sale or distribution that might be more stringent than those imposed by local, regional, or national regulatory authorities.
Scope
Product regulatory compliance touches on every aspect of the product lifecycle (from concept to retirement) and for the entire value chain (from critical components suppliers to the end customers) and is an important and omnipresent function impacting all other functions and stages (see Figure 1).
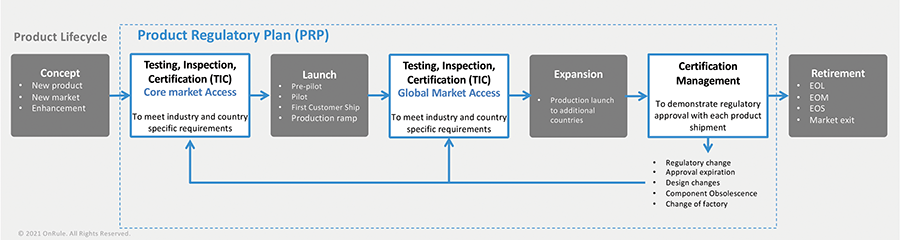
Concept to Launch
In the NPI phase, a product design is validated for product regulatory compliance through the testing process. Design-related issues, weaknesses, and defects identified during the testing of early prototypes are then incorporated into the next iteration of the product design to make the product more robust and compliant. Testing of the final product is then conducted to produce the test reports that are submitted to the relevant authority to obtain regulatory approval. Once approval has been received, the product is ready for general availability and for release in those core market(s) where approvals have been granted.
This process usually follows the following trajectory:
- Core Market Access (CMA): Companies generally first launch new products into their core or primary markets. Meeting the relevant compliance and testing requirements applicable in the EU and/or U.S. and Canada helps to validate the integrity of the product while also establishing a generally accepted baseline conformance to compliance.
- Global Market Access (GMA): Following a successful launch and acceptance of its new product into its core markets, companies then launch their product globally into other countries in succession. Some countries, such as China and India, typically require in-country testing, which requires shipping product samples to a local testing lab for the purpose of testing. Even in those countries where in-country testing is not required and in which regulatory authorities accept test results and approvals obtained in the EU or the U.S., companies may still be required to go through a time-consuming regulatory process, submitting applications and other forms to the relevant government agencies.
Launch to Retirement
Once a product has been released into production, it enters the sustaining mode. To support the growth of sales in new markets, fulfillment of testing and certification requirements applicable in additional countries is required. Global market access (GMA) is achieved through the fulfillment of the testing and certification needs of additional countries. In this phase, certification management of a company’s current product portfolio becomes critically important, and several events can occur that require a company to review existing product certifications for continued compliance. These events can include:
- Changes in an underlying standard (or standards) in a given country or jurisdiction may require retesting of the product to the new or revised requirements to either retain approval or receive a new approval.
- In cases in which a country or jurisdiction does not grant lifetime approval for a given product, renewal of an existing certification may be required. Typically, the frequency of such renewals can be anywhere from one to five years from the original approval date.
- A significant change in an existing product design can trigger the need to retest and/or recertify the product.
- A change in a critical component such as a power supply may also require a company to retest and/or recertify the end product.
- In cases in which regulatory authorities require follow-up inspections of the factory (or factories) where a product is produced, the use of a new or different factory may require a review of current product certifications and retesting.
As mentioned earlier, product regulatory compliance is evaluated at the product or SKU level. However, an OEM is required to disclose the list of critical components used in the final product. As part of the overall evaluation of the end product, some regulatory authorities may require evidence of safety testing and certification of those critical components. If a critical component is sourced from more than one supplier, (typically is the case for the purpose of managing the supply chain risk), evidence of safety testing and certification from all suppliers may be required.
Importance
If a product’s compliance with applicable regulatory requirements cannot be demonstrated, a company may be legally prohibited from shipping that product to their customers and may risk seizure of their product by customs officials at border crossings. This is not an uncommon occurrence, and many regulatory compliance engineers experience this situation multiple times during their careers.
Product regulatory compliance requirements play a significant role in your ability to ship your company’s products to foreign and domestic customers. Having sufficient evidence demonstrating your product’s compliance with applicable regulations to support a factory audit or to accompany your product when shipped requires verifying the validity, quality, and availability of your regulatory compliance documentation. Organizing that documentation and designating a secure location for it is also an obvious and commonplace practice that is essential to support the uninterrupted shipment of goods.
Engineering, NPI, and Product Management
During the new product development process, the product regulatory function must provide guidance to the design engineers as to the particular technical requirements that will apply to that product. The individuals or team responsible for product regulatory compliance should develop a test plan and testing methodologies to assess the new product. Doing so will help sharpen everyone’s focus on the particular regulatory requirements that will apply to the product when formal regulatory testing is conducted. It can also help the compliance team understand the potential compliance issues that might arise during the design and testing phase. Providing this guidance at an early stage in the product development process can reduce or prevent time-consuming iterations of the product design itself in order to comply with regulatory requirements. This early involvement in the design can also help the product to be designed so that the technical boundaries affecting the performance and safety around many critical parameters are taken into account.
In many cases, early testing on product prototypes against the limits set forth in applicable technical standards will pinpoint issues that may lead to non-conformity. Waiting until the product design has been completed to conduct testing almost always results in the need to redesign the product and to conduct regression testing on the updated design to verify its compliance. This inevitably leads to delays in bringing your product to market and increases the overall development cost for the product.
Product Management and Marketing
Obtaining product regulatory approvals is typically the last step before a product launch and represents a critical milestone in the NPI schedule. By this point, your product management and marketing teams should have a clear plan for the markets in which they want to launch the product, including a country-by-country sequence for market deployment. It is extremely difficult to launch a product in all targeted global markets in the same time period due to variations in the approval process among individual regulatory authorities in different markets and the amount of time required in individual jurisdictions. This is why a global product rollout is generally broken into different market segments to provide staggered availability dates for the product.
The best approach involves the development of marketing waves, that is, segmenting individual countries into groups to be given priority in the initial product rollouts to customers. The success of this wave approach ultimately requires the product regulatory group to develop a clear plan that accurately accounts for the time required for the testing and approval phases in individual jurisdictions so that product approvals coincide with the planned market availability. This regulatory compliance plan should be fully transparent to the entire product development team and the marketing team so that the necessary distribution channels can be established or verified as operational.
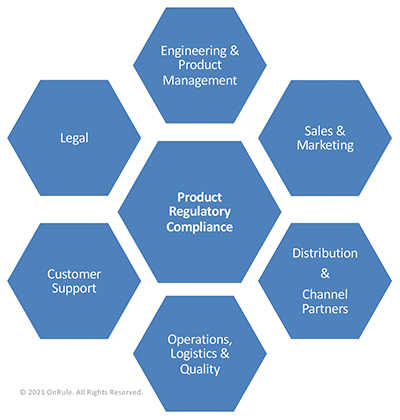
Sales
The ability to sell any new product depends on obtaining the required regulatory approvals to ensure the product’s legal availability to customers. The order management process in place in most companies typically will not authorize acceptance or shipment of an order for a new product until the required regulatory approval has been secured. Further, selling a new product in a new country or a region will first depend on the company’s ability to secure regulatory approval for the product in that country.
Distributors and System Integrators
Conforming with regulatory compliance requirements and obtaining independent verification of compliance is the responsibility of the OEM. Third-party distributors and system integrators who have a presence in a local market or country are often required to serve as an importer of the product into that country. As a result, most third-party distributors typically require documented proof of compliance before assuming responsibility for making a company’s product available to their end customers through their distribution channels.
Operations, Logistics, and Quality
The product regulatory compliance process is often part of the quality metrics that are presented during the product readiness review that takes place before the launch of a new product. At the same time, at the point of shipment, operations and logistics personnel need the required compliance documentation (approval certificates, manuals, packaging labels, etc.) in hand to avoid having products held up or denied at customs checkpoints or delayed at shipping docks and failing to reach the end customer as promised.
Customer Support
Existing end customers, channel partners, and field sales personnel will often contact customer support teams for compliance documentation that verifies that the requirements of the product regulatory approval process have been met. In some cases, this request can be for the approval documentation required for spare parts and critical components scheduled for shipment by the service department to existing customers.
Legal
Lastly, in several companies, the legal department gives the final nod to the product launch upon reviewing the completion of all regulatory milestones. Evidence that the product regulatory compliance process has been completed and that regulatory approval has been received is one of the important metrics reviewed and signed off by the legal team. In some companies, the product regulatory compliance team reports to the legal department.
The Impact of Non-Conformance
The failure to ensure product conformity with regulatory compliance considerations may have important impacts on several fronts, including on communities and on the business. Here are just some examples.
- Safety and well-being: A lack of conformity may result put the safety and well-being of people at risk. According to the CPSC, in 2019, there were an estimated 22,500 treadmill-related injuries treated at U.S. emergency departments among all ages (of which around 2,000 were children under eight years of age).4
- Revenue impact: It is all too common for companies to miss quarterly or annual revenue targets because they could not ship orders on hand from available inventory due to a lack of compliance documentation availability. We know of incidents in which a distributor could not bring inventory into a target market because the products were being delayed at customs due to a lack of compliance approval documentation. As a result, the OEM could not register the necessary revenue recognition during a fiscal quarter, falling short of both the company’s and investors’ expectations.
- Customer satisfaction: Missing an order commitment date due to lack of regulatory approval directly impacts customer satisfaction, a critical metric to business success. Note that an OEM customer may be a reseller, distributor, system integrator, or end customer.
- Brand impact: Recalls from the market due to poor quality and performance may adversely impact the OEM brand. The bad press from an incident can be devastating to a company’s reputation, from which it may never recover. In the infamous hoverboard issue cited previously in this article, more than ten companies were forced to recall 100,000 hoverboards after the CPSC received about 100 reports of the lithium-ion battery packs that power hoverboards overheating, sparking, smoking, catching fire, or exploding.
- Legal impact: Typically, good business practices dictate that a company secures product liability insurance prior to placing the product on the market. Generally speaking, product liability insurance premiums for a product that meets all of the applicable regulatory requirements will be significantly less than that paid for a non-compliant product or a product that has no proof of compliance. If a non-compliant product is introduced into a market and an event occurs that brings a safety issue to light, there can be many ramifications that directly affect the company, including fines and possible jail time for those responsible.
Conclusion
The emergence and importance of product regulatory compliance as a formal discipline in governing and ensuring the release of safe, environmentally sustainable, and energy-efficient products in the global markets have now been established and recognized. CE, FCC, UL, etc. marks are understood and regarded by consumers and businesses at large. But the scope and impact of product regulatory compliance in safeguarding our universe, planet, and human beings through the introduction of compliant products are much broader and deeper. Products, whether used underwater, on earth, or in space, are all subjected to and benefit from this ubiquitous discipline. As technologies evolve and as mankind races to explore far space, it is imperative that this discipline be further promoted, developed, and implemented.
References
- https://www.cbsnews.com/news/e-scooter-hoverboard-e-bike-deaths-41-last-3-years by Kate Gibson, September 17, 2020, CBS News, Moneywatch.
- https://www.cpsc.gov/Recalls/2016/self-balancing-scooters-hoverboards-recalled-by-10-firms
- https://www.a2la.org/regulators/domestic-recognitions
- https://abcnews.go.com/US/consumer-regulatory-agency-issues-urgent-warning-peloton-treadmill/story?id=77138617#:~:text=In%202019%2C%20there%20were%20an,5%2Dyear%2Dold%20child
