Associate Professor Neils Jonassen authored a bi-monthly static column that appeared in Compliance Engineering Magazine. The series explored charging, ionization, explosions, and other ESD related topics. The ESD Association, working with In Compliance Magazine is re-publishing this series as the articles offer timeless insight into the field of electrostatics.
Professor Jonassen was a member of the ESD Association from 1983-2006. He received the ESD Association Outstanding Contribution Award in 1989 and authored technical papers, books and technical reports. He is remembered for his contributions to the understanding of Electrostatic control, and in his memory we reprise “Mr. Static”.
~ The ESD Association
Reprinted with permission from: Compliance Engineering Magazine, Mr. Static Column Copyright © UBM Cannon
The damaging effects of static charges on insulators can be reduced or even negated.
The first of this two-part series (“Abatement of Static Electricity – Part I: Conductors,” In Compliance Magazine, June 2013) covered the abatement of static charges on conductors. This second installment addresses charges on insulators, which must be neutralized differently than charges on conductors.
In principle, there are three methods for neutralizing charges on insulators: conductance through the bulk of the material, conductance along the surface of the material, and the attraction of oppositely charged ions from the air.
Bulk Conductance
If a material contains mobile charge carriers, it is said to be bulk conductive. If a field strength E in the material releases a current density j, the bulk conductivity g of the material is defined by
j = γE (1)
or, as it is usually written,
E = ρj (2)
where r = 1/γ is the bulk resistivity. These equations are forms of Ohm’s law. It appears from Equation 2 that the unit for r is (V/m)/(A/m2) = Ω ∙ m.
Figure 1 shows a material, A, with bulk resistivity ρ and relative permittivity εr. “A” is resting on a grounded plate, G. If A is charged with a surface charge density σ, a field E is established in A and is directed toward G. It is assumed that all the field lines (the total electric flux) from the charge run through A (i.e., the field outside A is negligible). This field makes positive charge carriers move toward G and negative charge carriers move toward the surface of A, eventually neutralizing the field from the original charge.

Figure 1: Material A has bulk resistivity r and relative permittivity εr.
Charge density σ appears to decay through material A according to the equation
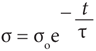 (3)
(3)
where so is the initial charge density,
t = εrεoρ, (4)
is the time constant, with εo = 8.85 × 10–12 F ∙ m–1. It is therefore possible from the measurement of material parameters r and er to predict how fast a surface charge is being neutralized. The question is then how to make insulators bulk conductive.
Bulk Conductive Insulators
It is contradictory to talk about transporting charges through an insulator. If this were possible, the material would not really be insulative. Over the years, many attempts have been made to give insulative materials a suitable conductivity without ruining their other (usually mechanical) desirable properties. Normally, this is done by mixing the material with inherently conductive additives. The best-known example of such an intrinsic antistatic agent is carbon black. Carbon black can be added to a variety of polymeric materials and is used when the resulting blackening of the base material is acceptable.
For many years, the most important area of use for carbon black was conductive rubber. Ordinary vulcanized rubber can have a bulk resistivity of 1013 Ω ∙ m, but adding carbon black can lower the resistivity by a factor of up to 1015. Normally, however, a resistivity of about 105–106 Ω ∙ m is low enough to prevent dangerous or annoying charge accumulations.
Conductive rubber is used extensively in hospital operating rooms, tubing for anesthetic machines, wheels on carts, soles for antistatic footwear, and car tires. It should be mentioned that the shock a driver or passenger can receive when getting out of a car is not caused by discharging the car to ground. Instead, the driver may get charged when sliding over the seat cover, in much the same way a person gets charged when getting up from a chair with an insulative seat. As a result, a spark can jump between the person and any metal part of the car, which is virtually at ground potential.
Another use of carbon black is in the manufacturing of solid and textile antistatic floor coverings. The textile fibers can be made with either a central core of carbon black and a sheath of polyamide or, conversely, with a central core of polyamide and a sheath of carbon black.
The most important use of carbon black, at least economically, is no doubt in the electronics industry. By loading the base materials for carrier trays, holders, tubes, tote boxes, bags, etc., with carbon black, these items are made sufficiently conductive to ensure a rapid neutralization of static charges on the material itself. Usually, the loading is done uniformly throughout the matrix of the material to increase the bulk conductivity, but it can also take the form of a thin conductive surface layer.
Surface Conductance
In many static-electric processes, it appears that not only the charge separation but also the subsequent charge neutralization takes place in or along the surfaces of the materials involved. It may therefore seem practical to define quantities similar to the bulk parameters of Equations 1 and 2 to characterize a surface’s resistive properties.
If a field with strength Es along a surface releases a current with linear density js, the surface conductivity gs can be defined by
js = γsEs (5)
or, as it is usually written,
Es = ρsjs (6)
where ρs = 1/γs is the surface resistivity. Because js is a linear current density with the unit A/m, it appears from Equation 6 that the unit for ρs is (V/m)/(A/m) = V/A = Ω. Equations 5 and 6 both express Ohm’s law for surface conductance.
Knowledge of the bulk resistivity (and the permittivity) can be used to predict how quickly a charge is neutralized by conductance through the bulk of a material (see Equation 3), as long as the field from the charge runs primarily in the material itself. This condition is often fulfilled with sufficient accuracy with bulk conductance, but rarely with surface conductance.
Figure 2 shows an insulative material, A, on top of which is a thin conductive layer, B. A grounded electrode, C, is placed in direct contact with one end of B, and a positive charge q is placed on the other end of B. If C is the only conductive grounded item near the system, all the field lines from q will eventually end at C. The parts of the field lines running through B will cause negative charge carriers to move toward q and eventually neutralize it. However, the field running through the insulator A or though the air does not contribute to the neutralization process at all.

Figure 2: Insulative material A with conductive layer B
Surface-Conductive Insulators
It is well known that static-electric problems seldom occur in environments with high relative air humidity, say, greater than 50–60%. This fact has sometimes been erroneously interpreted to mean that humid air has higher conductivity than dry air. However, if anything, humid air is less conductive, because the mobility of small air ions decreases slightly with increasing humidity. The effect of increased air humidity is to increase the thickness of the moisture layer on or in all surfaces, and this layer contains electrolytic ions that provide neutralizing charges.
The amount of moisture absorbed or adsorbed from the air is strongly dependent on the material in question. At humidities as low as 30–35%, a material like cotton may show little charge retention, whereas a material like polyamide may require humidities of 50% or greater to be considered antistatic. Generally speaking, no resulting charges appear at humidities of 60% or greater. Humidities at such high levels, on the other hand, often pose practical, technical, or hygienic problems if they are maintained over extended periods.
Topical Antistats
It is often possible to render highly insulative materials sufficiently surface-conductive, even at relatively low humidities, by treating the surface with antistatic agents (topical antistats). These agents function by forming a surface layer a few molecules thick that attracts moisture from the air much more readily than an untreated surface.
Antistatic agents obviously must be hygroscopic, but they also must show a low vapor pressure in order to keep from evaporating too quickly from the treated surfaces. Further requirements concern color, toxicity, inflammability, etc.
Chemically speaking, antistatic agents are amphipathic compounds, their molecules containing a hydrophobic group to which is attached a hydrophilic end group. According to the nature of the end group, the agents are divided into cationic, anionic, and nonionogenic agents. Cationic materials are usually high-molecular quaternary ammonium halogenides or ethoxylated fatty amines or amides. Anionic materials can be sulfonated hydrocarbons, and nonionogenic materials can be polyalkylene oxide esters.
Topical antistats are used extensively in the textile, plastic, and printing industries. A common use is the treatment of floor coverings to reduce the body voltage of persons walking across the floor. With textile floor coverings, a proper antistatic treatment may be effective for two to three months. With hard floor coverings, the antistatic treatment must normally be repeated after each washing.
Permanent Antistatic Materials
In some cases, antistatic agents may be compounded with a polymer, either before polymerization or at least before extrusion. The best-known example of this technique is probably the manufacture of antistatic polyethylene, commonly known as pink poly. Ethoxylated fatty amines or amides are mixed with a resin, such as low-density polyethylene, and an antiblock, such as calcium carbonate, to prevent stickiness. After extrusion or molding to the required end product (film, sheets, trays, boxes, etc.), the additive has to diffuse (bloom) to the surface to attract moisture from the air and thus render the material antistatic.
Pink poly, which may appear in a variety of color shades besides pink, is no doubt the most widely used material in the electronics industry for packaging, storing, and transporting sensitive components and circuits. Materials with built-in additives maintain their antistatic properties as long as the additive is present on the surface.
Although the vapor pressure of most additives is fairly low, a certain level of evaporation always takes place from the surface. For fresh materials, this evaporation is counterbalanced by diffusion from the interior of the material. As the supply of additive in the solid is depleted, the surface concentration cannot be maintained. The surface is said to “dry out,” resulting in an increasing surface resistivity and the eventual loss of antistatic properties.
The effective lifetime of a permanent antistatic material depends on many factors, the most important of which are the temperature of the environment and the thickness of the material, which (for a given volume concentration) determines the amount of additive available for diffusion to the surface. It should also be mentioned that the additive diffusing to the surface, besides attracting moisture from the air, may react in unwanted ways with components and devices coming into contact with the material. Such unwanted reactions include printed circuit boards and other items made of polycarbonate crazing and cracking when packed in antistatic materials containing fatty amines.
Charge Neutralization by Air Ions
In all the methods discussed above for neutralizing charges on insulators, some kind of modification of material parameters, such as surface or bulk resistivity, is involved. However, such modifications are often neither possible nor acceptable. In such cases, only one method remains: Neutralize the charges with oppositely charged air ions. In a previous article (“Ions,” In Compliance Magazine, November 2011), the physical properties of air ions and their formation were discussed. This article concentrates on the processes of charge neutralization.
The charge carriers, either electrons or electrolytic ions involved in bulk and surface conduction, are fairly stable quantities that are always present and ready to move when exposed to a field from a charge. The neutralization processes do not change the concentrations, and negative and positive (electrolytic) ions exist side by side without trying to annihilate each other. In some cases (bulk conduction), it is even possible to predict how fast a charge is being neutralized.
However, this is not so with air ions. First, air ions are not naturally present where they are to be used, except in environments with high radon and radon-daughter concentrations. They must be produced (by high electric fields or radioactive decay) somewhere else and brought to the charge by a field, sometimes aided by airflow. Furthermore, air ions are unstable structures with a limited lifetime. Whereas a stable, high bulk or surface conductivity can be created in or on suitable materials, this is not the case with air ionization.
Suppose a high density of ions is created in a room with comparable concentrations of positive and negative ions: The ions will disappear if there is no supply of new, freshly formed ions. The ions disappear by combining with airborne particles; by positive and negative ions recombining and turning into oxygen, nitrogen, and a few water molecules; or by plating out on any surface in the room.
Despite the apparently negative qualities of air ions, the use of air ions is the only way to neutralize charges on insulators.
Air Conductivity and Resistivity
Air containing ions is conductive in a way similar to solid materials containing mobile charge carriers (see Equations 1 and 2). However, when dealing with air ions, conductivity caused by negative ions and conductivity caused by positive ions must be distinguished from each other.
In an atmosphere with positive and negative ions, an electric field E will cause a current with density j+ in the direction of E,
j+ = γ+E (7)
where g+ is the conductivity caused by positive ions (positive conductivity).
Equation 7 can be rewritten as
E = ρ+j+ (8)
where ρ+ = 1/γ+ is the positive resistivity of the air.
The same field E also causes a current (carried by negative ions) with density j– in the direction opposite to that of the field, giving
E = p− j− (9)
where p− is the negative resistivity of the air.
Figure 3 shows a positively charged insulator, A, in an atmosphere with positive and negative ions. The positive ions are repelled (as long as A itself is positively charged) and therefore have no influence on A’s charge.
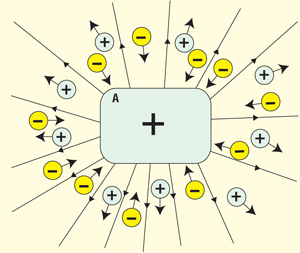
Figure 3: Positively charged insulator in ionized atmosphere far from grounded objects
The negative ions, on the other hand, are attracted toward A and plate out on the surface. Whether the charge of the negative ions actually neutralizes the positive charge on A or the field from the plating-out ions just superimposes the field from the charge on A is a question of academic interest. The result is that A appears to gradually lose its charge. If the field from A (the flux) extends mainly into an atmosphere with negative resistivity p−, the charge q+ on A would appear to decay according to the equation
 (10)
(10)
where qo+ is the initial charge and τ+ is the time constant for positive charge decay given by
τ+ = εoρ− (11)
It therefore appears that the neutralization rate for a positive charge is determined by the negative resistivity of the surroundings, or, more precisely, by the resistivity caused by the negative ions.
Equations 10 and 11 are parallel to Equations 3 and 4 for bulk decay through a solid material, but it should be stressed that a time constant calculated from Equation 11 is usually lower than what can be found experimentally. The reason for this is that a charged body is rarely far from other bodies (especially conductors), as assumed in Figure 3.
The situation in Figure 4 may be closer to reality. Here, the charged insulator, A, is placed close to a grounded conductor, B, maybe even touching it. Parts of the field lines from A terminate on B and run through a space with no or very few ions. This part of the field does not contribute in full to the neutralization, and, consequently, the process is slower than if A had been suspended freely in an ionized atmosphere.
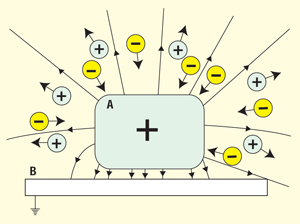
Figure 4: Positively charged insulator in ionized atmosphere near grounded conductor
This situation corresponds rather closely to the situation illustrated in Figure 2 for surface conduction. There is, however, a major difference. Whereas a surface decay time cannot be calculated or predicted and can hardly be measured, the neutralization time by air ions can often be accurately estimated with a charged-plate monitor or similar instrument.
Ionizer Types
Any ionization process in air starts with an electron being knocked off an oxygen or nitrogen molecule. This process is done in different ways in radioactive ionizers and field ionizers.
Radioactive Ionizer: A radioactive material (typically an alpha-emitting nuclide with a half-life on the order of half a year) is placed on a base material and covered by an extremely thin protective layer, often made of gold.
The alpha particles are emitted from the nuclide with an energy of, say, 5 MeV (» 8 x 10–13 J). A small part of this energy is dissipated in the protective layer, but the alpha particle is still able to create maybe 150,000 positive and negative ion pairs along its range of a few centimeters.
The ionizer is consequently placed in front of the charged material at a distance that is a little farther than the range of the alpha particles. If the material is positively charged, negative ions will be attracted and plate out on the material, gradually reducing the field.
The neutralizing efficiency of radioactive ionizers is not very high, but with relatively low levels of static charges and especially in confined spaces, radioactive ionizers are very handy. They do not require any electrical installation, and they cannot cause potentially harmful electrical discharges.
Because a fairly short-lived nuclide is used, the ionizer is replaced at regular intervals and not left unattended for extended periods. Because alpha-active nuclides are used, the external radiological dose is insignificant. However, if the radioactive material is accidentally spread into the environment and becomes airborne, it can be inhaled. In this case, the highly energetic alpha radiation may give off an internal dose which can eventually cause radiological damage to the respiratory tract. With modern ionizers, however, the risk is extremely low.
Field Ionization: In a radioactive ionizer, alpha particles are emitted with sufficient energy to cause ionization of a large number of air molecules. In the more commonly used electrical or field-based ionizers, the necessary energy is delivered by accelerating an electron in a strongly inhomogeneous field.
Figure 5 shows a point electrode, a so-called emitter. If the electrode is kept at a sufficiently high potential with respect to grounded surroundings, the field strength E in the immediate neighborhood of the electrode will exceed the breakdown field strength Eb.

Figure 5: Field ionization
In this range, positive and negative ions are formed. If the emitter is positive (see Figure 5), the negative ions will move toward the emitter, where they will be neutralized, delivering their negative charge to the emitter. Accordingly, the positive ions will move away from the emitter, making it look as if the emitter has indeed emitted positive ions. But it hasn’t. The emitter does not emit anything. The ionization process takes place exclusively in the air in front of the emitter. In addition, the ionization is not caused by the voltage of the emitter but by the field.
Passive Ionizer: The simplest form of field ionizer is a passive ionizer. It is essentially a single grounded emitter or (more often) a row of grounded emitters placed parallel with and close to the charged material. The charge provides an electric field. If the charge density is high enough, the breakdown field strength is exceeded near the emitter and positive and negative ions form in the region. Negative ions move to the emitter and become neutralized, and positive ions move to the charged material and gradually neutralize the charge located there.
When the charge density becomes too low, the ionization stops; hence, the neutralization stops. A passive ionizer will therefore not be able to render a material totally neutral, but it will be able to reduce high levels of charges, which in many industries is sufficient.
It should be stressed that the emitter should not touch the charged material. The neutralization is not caused by contact but by the ionization process.
Ac Ionizer: In cases where a passive ionizer does not provide sufficient neutralization, an ac ionizer can often do the job. The emitter is connected to an ac voltage supply, usually in the kilovolt range. In front of the emitter, the formation of positive and negative ions alternates, and the polarity of the charged material determines the polarity of attracted ions.
It is a shortcoming of ac ionizers that ionization only happens in that part of each half-cycle when the voltage of the emitter exceeds the breakdown voltage. Therefore, if the charged material is moving rapidly past the ionizer, neutralization can be incomplete. Furthermore, the ac signal should not be symmetrical—the breakdown voltage is lower for negative ionization than for positive.
Dc Ionizer: The most effective neutralization is obtained by the use of a dc ionizer, which usually consists of two emitters held at a positive and a negative potential, respectively (see Figure 6).
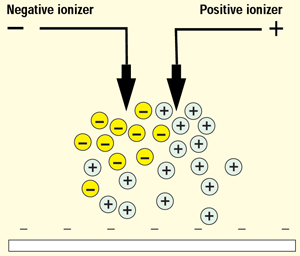
Figure 6: Dc ionizer
When the ionizer is properly balanced, positive and negative ions are provided in the same concentrations in front of the charged material, and, as explained for the ac ionizer, the polarity of the charge determines the kinds of ions used for neutralization.
If the charge to be neutralized is always of the same polarity (for instance, the negatively charged material in Figure 6), it might seem natural to use only a positive dc ionizer. This, however, may not ensure neutralization but instead may lead to a positive charge caused by the ionizer. It is therefore important that the ionizer be able to balance the ion concentrations where the neutralization will take place.
General Remarks on Ionization and Ionizers
Practical, commercial ionizers do not look much like those shown in Figures 5 and 6. Often, they are mounted in front of a fan to propel the ions to where they are needed. Such ion blowers are handy for localized neutralization.
If it is necessary to secure neutralization in larger areas or in larger volumes, whole-room ionization may be employed. In such systems, a number of ionizers are mounted beneath the ceiling. Emitters can alternate positive and negative or all can be connected to an ac voltage, either sinusoidal (50 or 60 Hz) or square-pulsed (1–2 Hz). With the square-pulsed technique, ions with alternate polarities are constantly produced, and, because the pulses are fairly long, ions of a given polarity have a chance to move away from the emitter before ions of the opposite polarity are produced and recombination sets in. Separating shorter positive and negative pulses by half a second (or so) in the stepped-pulse technique can enhance the process. The ions are carried to workplaces and items where neutralization is needed by fields, diffusion, and, most often, by laminar airflow.
Conclusion
This series of articles is not intended to be a handbook on fighting the risks and nuisances of static electricity. Rather, it is meant to give an overview of the various ways of attacking these problems and, to some extent, to describe the pros and cons of implementing different methods. ![]()
 |
Niels Jonassen, MSc, DSc worked for 40 years at the Technical University of Denmark, where he conducted classes in electromagnetism, static and atmospheric electricity, airborne radioactivity, and indoor climate. After retiring, he divided his time among the laboratory, his home, and Thailand, writing on static electricity topics and pursuing cooking classes. Mr. Jonassen passed away in 2006. |
