• Product Safety
• Electromagnetic Compatibility (EMC)
• Homologation of wired and wireless telecommunication devices
• Energy Efficiency
• Environmental
• Chemical
Such regulations are established at many levels, including national, regional, state, province and even individual cities or towns. In many cases, hardware regulations carry the force of law. Hence, a complete and in-depth understanding of the regulations applicable to any particular product is needed to avoid running afoul of the law. Being aware of all the regulations that apply to a product can be challenging enough, even before understanding all the details.
Regulatory Fundamentals
Regardless the discipline, all hardware regulations encompass a common set of basic elements.
- Technical evaluation, which may include testing or engineering analysis
- Documentation of results, often in the form of a test report
- Conformity assessment procedures, including Declaration of Conformity (DoC), verification and certification
- Product and packaging marking
- Information to the user, with required language translations
- Market surveillance and on-going compliance
- Registration to the government and follow up (registering laser devices with the FDA, for example)
It should be noted that some regulations may not require explicit action on all of these elements. For example, certain regulations do not require a statement of compliance to be included in the documentation provided to the end user of the product. Other elements may be included as well, such as an audit of procedures and capabilities of manufacturing factories.
The technical evaluation typically includes either testing a sample of the product against some defined standard or set of standards or an engineering analysis or assessment. Restrictions or rules on who can perform the testing or evaluation vary. In some cases, the test or assessment may be performed by the product’s manufacturer, while other regulations for the same basic discipline may require the use of an independent third party. If testing to standards is required, the lab performing the testing may need to be approved by the regulatory agency or accredited through a designated lab accrediting agency. With the wide possibility of requirements on who can perform the evaluation and what specifically is required or allowed, it is easy to see why in-depth knowledge of the applicable regulations is essential for successful compliance.
Once the technical evaluation is completed, the results must be documented. The old adage of the work not being done until the paperwork is completed definitely applies in hardware compliance. Without adequate documentation of the evaluation, one cannot truly demonstrate compliance with the requirements. What product was evaluated/tested? What configuration was evaluated/tested? How was the evaluation/test performed? Who did the work, and were they properly qualified to do it? If the company is accredited to perform the work, is their accreditation through an organization that is accepted or recognized by the regulatory body? The list of content that must be included in a test report can be quite extensive. Consider the following example.
- Test Report Cover Page stating the regulation the report encompasses
- Test standard and test method that were applied and any deviations from the specified procedures
- Classification of the product with respect to the regulation (for example, Class A or Class B for EMC emissions test results)
- Description of the device being tested for approval, including marketing designation or model number
- Product specification sheet describing its functions and capabilities
- Functional block diagram
- Specific identification of the device that was tested, including serial number and detailed list of all hardware content
- Description of software used to exercise the unit being tested
- Measuring equipment used in performing the test, including make, model, serial number and calibration details
- Test results
- Description of any changes made to the device during testing to meet the test limits
- Photographs of the test setup
- Photographs of the device being tested
- Diagram of the physical arrangement and configuration of the unit tested
- Drawing or photograph of the product label showing required marking(s) and location of label on the device
The conformity assessment procedures define the specific process steps that must be followed to satisfy the regulation and include things such as filing a report with an agency versus keeping it on file to be made available if requested. These procedures can be placed into three basic categories:
- Certification
- Suppliers Declaration of Conformity
- Verification
Certification generally requires filing specific documentation with the agency and receiving a certificate in return. Required documentation may include a test report or detailed technical assessment of the equipment being certified, description of the equipment, instructions furnished to the user of the equipment and information about the manufacturer importer. To ensure the manufacturer does not swap parts for inferior/lower cost substitutes after the product is certified, all components that are considered critical are included in the certification documents. Agency representatives may audit the manufacturer to verify the product continues to be built as originally tested. Quarterly audits are a common practice for Product Safety certifications. Changes in the hardware considered critical would drive recertification.
In a Suppliers Declaration of Conformity procedure, the supplier (typically the product’s manufacturer) completes a form attesting, or declaring, that the device complies with the required regulation. The method used for demonstrating compliance is often listed on the declaration. In some cases, the declaration is distributed with the product to the end user; while in other cases, it is kept on file to be made available upon request.
Verification is the simplest form of conformity assessment in which the supplier creates documentation to verify that the product meets the requirements. Typically, this documentation would be a test report that is kept on file and made available upon request.
Laws and regulations from different countries drive the manufacturer to choose the specific method that will make marketing a product feasible. In the USA for example, OSHA clause 1910.399 and national Electric Code (NEC) clause 90.7 drive the IT industry into using the certification method. In the European Union, DoC is widely accepted. In terms of cost and work by the manufacturer, the DoC would be considered the preferred methodology as it eliminates the third party certification agency. Certification via a third party agency adds delays to the certification cycle, it relies on the availability of the agency and adds costs for initial certification, manufacturer’s audits, recertification and annual fees to place the agency’s mark on the product. DoC requires all the documentation to be available; so, the manufacturer would still be required to do all the testing and keep the documentation on file.
Product marking involves placing a mark or statement on the product. Product’s information labels are usually used as a venue to distribute regulatory information. Some of that information includes the following items.
- Trademark, Model Designation, Certification Marks and Statements: Information on product certificates should match information provided on the label. For rebranding agreement, where the company’s trademark shown on the label does not match the original manufacturer (owner of the certification), a number may be included below a certification mark, which can be used to relate the product back to the original manufacturer.
- Manufacturing Location and Country of Origin: This information, historically required for trade and customs, is becoming more and more important in the regulatory area, because some countries are starting to require certification is done per manufacturing site. That means, when submitting a sample for testing, assuming you have two potential manufacturing locations, you would need to submit samples from each location to be able to ship from both manufacturing locations.
- Translations: For certain categories of products, specific information on the label needs to be translated. Figure 1 is an example where both simplified and traditional Chinese text are included, to meet requirements for China and for Taiwan.
Figure 1: Product rating information shown with required translations of test into simplified Chinese and traditional Chinese
Different regulations require the marking is placed on the product or on both the product and the packaging. Other regulations allow alternatives of placing the product marking on the packaging or in the user manual.
Information to the user is generally a statement that the product complies with the regulation. It may also include caution or warning statements describing types of locations where the device is, or is not, allowed to be used. This information may have to be provided in more than one language. For example, in Canada, text-based statements targeting the end user have to be provided in both English and French.
Market surveillance includes any activities undertaken by the authorities to verify that products being sold do, in fact, comply with all applicable regulations. Market surveillance activities take many forms and may include checking products at retail outlets to ensure proper labeling; requesting copies of test reports, DoCs or certificates from the manufacturer or importer; or performing the tests defined by the standards or regulations on samples acquired from manufacturers, importers or retail outlets.
Compliance verification by Customs officials at the time of importation is another form of market surveillance. Verification by Customs typically involves document inspection to see if all the paperwork accompanying a shipment is in order. Noncompliances discovered during Customs verification typically result in delayed product deliveries to customers, as the noncompliant product (or suspected noncompliant product) will likely be held by Customs until compliance can be demonstrated or obtained. Even simple errors in documentation, such as the model number shown on the commercial invoice not matching the information on the certificate issued for the product, can create problems at the time of importation. Therefore, attention to detail is very important. This practice seems to be gaining in popularity among national agencies. Recently, the Customs Union of Russia, Belarus and Kazakhstan announced their customs authorities would begin checking imports of equipment in certain product categories to verify compliance with the EMC requirements prescribed by the EuroAsian Economic Commission (EAC). South Korea has been executing a similar verification process for several years.
Let us now explore EMC regulations around the globe.
A device’s ability to exist in its intended operating environment without causing electromagnetic interference with other electronic equipment (emissions) or without suffering undue interference from other equipment (immunity) is regulated in some 50 countries.
Fortunately for manufacturers, importer and other responsible parties, these regulations reference a much smaller set of common standards, as shown in Table 1.
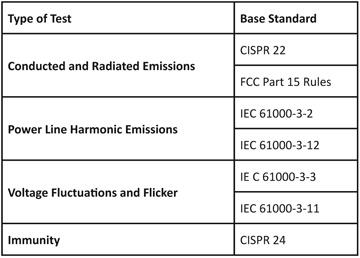
Table 1: Common standards serve as the basis for global EMC regulations
This referencing of common standards substantially reduces the testing burden, although changes and revisions to the reference standards are not always adopted on uniform schedules by the various regulations. A recent example of the variations that can happen in adoption is the roll out of the CISPR 22 limits on radiated emissions between 1 and 6 GHz. Compliance with these limits became mandatory in October 2010 for the Republic of China (Taiwan), in March 2011 for the Peoples Republic of China, and October 2011 in Australia, the European Union and Japan. Depending on the changes introduced in subsequent editions of a standard, the effect of nonuniform implementation schedules can range from simply referencing the correct edition in test reports to testing a single product multiple times to accommodate the technical differences between versions if the standard.
Now that the new CISPR 32 standard for emissions from multimedia equipment has been published, it will be interesting to see how the various jurisdictions incorporate the standard into their requirements.
Even with the use of these common standards to establish the test conditions and limits that must be met, the industry must understand and correctly apply differences in the conformity assessment details between various global EMC regulations. A sampling of these details is summarized in Table 2. Note that some regulations include multiple conformity assessment procedures, usually based on the type of product or product classification.
Table 2: Sampling of compliance details for EMC regulations
Conclusion
Many countries around the world have hardware regulations that must be met before ITE is marketed, sold or imported into those countries. These regulations exist for valid reasons and generally are intended to protect something: people, other equipment or the environment. Meeting the technical details of hardware regulations is only one step in satisfying the regulations. Satisfying the administrative elements of the conformity assessment process that need to be completed after the technical analysis or testing is finished can be more challenging and time consuming than the test or analysis itself.
Effective regulatory compliance engineers must have a solid technical background to understand the intricate details of product designs and the related test standards and evaluation criteria. They must also stay current on the ever-evolving test and analysis standards, related test equipment, laboratory performance and approval criteria, accreditation requirements, import rules and the rules for the declaration and certification regimes of multiple regulatory agencies throughout the world. These skills must then be applied with meticulous attention to detail.
| John Maas is a Senior Technical Staff Member and Corporate Program Manager for EMC at IBM Corporation, where he has responsibility for IBM’s worldwide EMC regulatory compliance programs. John has more than 30 years of EMC experience including hardware design and test. He is a senior member of the IEEE and has been involved in international standardization for much of his career, with his contributions to EMC standardization being recognized by the IEC when he received the IEC 1906 Award. John is currently convenor of IEC SC77B/WG10, Technical Advisor of the US technical advisory group (TAG) for IEC SC77A and a member of the US TAGs for IEC TC77, SC77B and CISPR/I. Mr. Maas can be reached at johnmaas@us.ibm.com. | |
| Mariel Acosta-Geraldino is the Corporate Program Manager for Product Safety at IBM, where she has the responsibility to ensure IBM products meet with any safety regulations in the Americas. She has been with IBM for 13 years. Her previous roles at IBM include Safety Team Lead for Storage, testing and ensuring products were safe and compliant and power supply qualification. Educational background includes M.S. Manufacturing Systems Engineering and B.S. Electrical Engineering. |