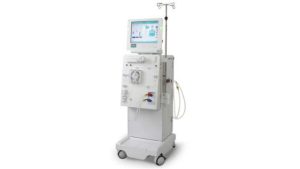
The Dialog+ Hemodialysis System is a machine used in the treatment of chronic kidney disease for patients whose own kidneys are no longer healthy enough to filter their blood of wastes and excess fluid. These systems are used in hospitals, health centers, and in outpatient dialysis center settings.
B. Braun Medical Inc. is recalling these devices because cracks in conductivity sensors may allow air to enter into the solution that is used to help filter waste and other excess fluids in the blood. The presence of air in dialysis fluid may lead to improper blood filtration, causing serious adverse health consequences, including death. Therefore, the FDA has identified this as Class I, the most serious type of recall. To repair the affected devices, B. Braun will send a qualified service technician to run a pressure test and replace the sensors as needed.